
When I was a kid, I loved watching fireworks burst, spin, and shimmer. Now that I'm older and understand the science behind this vibrant spectacle, I am even more awe. The precise chemistry, physics, and engineering required for these displays is incredible. This Independence Day is the perfect opportunity to spark (pun intended!) curiosity about how and why fireworks work.
Where Did Fireworks Come From?
Fireworks were first discovered over two thousand years ago in ancient China, likely by accident. Around the 9th century, Chinese alchemists discovered gunpowder which is a mixture of potassium nitrate, sulfur, and charcoal. When packed into bamboo tubes or paper casings, this “black powder” created bright flashes and loud explosions that were soon used in festivals to scare away evil spirits. Over time, these early fireworks evolved into more elaborate displays. By the 13th century, the knowledge of gunpowder and pyrotechnics had spread to the Middle East and Europe, where fireworks became popular in royal celebrations and military demonstrations. By the 18th century, chemists began isolating and identifying elements that burned in specific colors, such as copper for blue and strontium for red. This marked the beginning of fireworks chemistry in which display designers chose materials deliberately based on their chemical properties rather than tradition alone.
The real leap forward came in the 19th and 20th centuries, with advances in chemistry, materials science, and mechanical engineering. Scientists refined black powder formulations, discovered more reliable oxidizers and fuels, and developed stable color-producing compounds. Engineers began applying ballistics, timing mechanisms, and shell design principles to shape complex multi-stage fireworks and synchronized displays. The invention of electronic ignition systems and computerized firing sequences in the late 20th century transformed fireworks into a precise blend of art, chemistry, and physics.

How Do Fireworks Work?
Today, every firework is a carefully engineered system. From its chemical makeup and burn rate to its trajectory and timing. While the magic of fireworks still captures the imagination, it’s now very much rooted in science. Let’s explore the science behind fireworks in a way that’s fun, engaging, and easy to share with kids.
Fireworks begin with a carefully constructed shell that contains everything needed for a dramatic display. When a firework is lit, the first thing that happens is the lift-off. This is powered by a small explosive, often black powder, that launches the firework high into the sky. Once the firework reaches the right height, a time-delay fuse triggers the internal burst charge, which ignites a collection of small pellets called “stars.” These stars are the real showstoppers as each one is designed to produce light, color, and special effects when it burns.
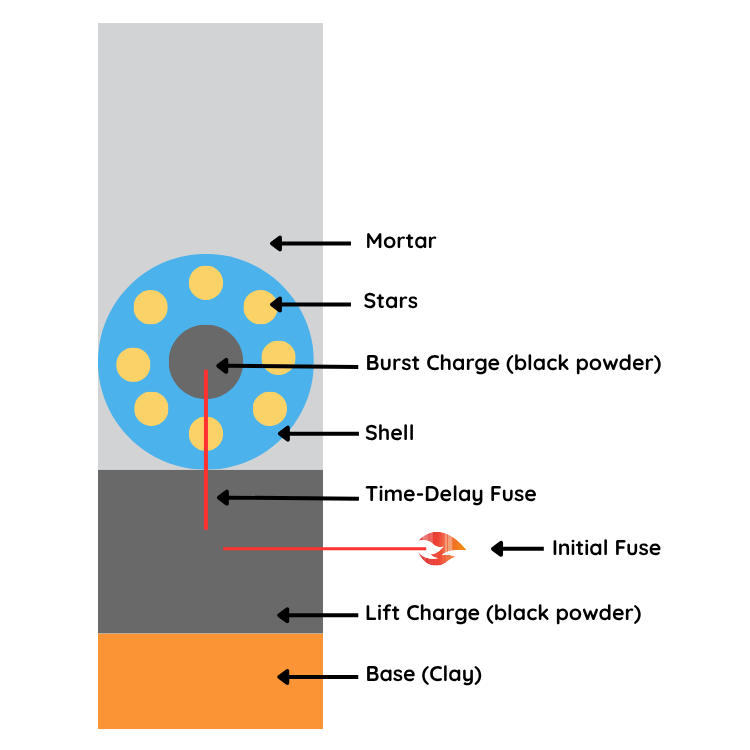
What Makes the Different Firework Colors?
The colors that paint the sky come from different metal salts packed into each star. When these metals are heated, they emit specific wavelengths of light. Strontium compounds burn bright red, while barium gives off a lush green. Copper produces a brilliant blue, although it’s one of the hardest colors to get just right. Sodium yields a vivid yellow, and calcium creates a rich orange. When pure metals like magnesium or aluminum burn, they produce the dazzling white or silver sparkles that shimmer so brightly. If you want to demonstrate this at home, safely burning a piece of copper wire (with full supervision and safety precautions) can show a bit of that green-blue color magic in action.

What Makes the Different Firework Shapes?
Beyond color, the stars are also responsible for the unique patterns you see in the sky. Their placement inside the firework shell and the way they burn and move after ignition are what determine the final visual effect. For example, in a peony firework, the stars are arranged evenly around the burst charge. When they’re ignited, they all shoot outward at the same time, creating a perfectly round bloom of color that quickly fades. A chrysanthemum uses the same spherical arrangement, but the stars are made with compositions that burn more slowly and leave glittering trails, giving the burst a flower-like appearance with glowing petals that linger.
In the willow effect, the stars are heavier and burn even longer. After the burst, they arc outward and downward in long, drooping trails of light, mimicking the weeping branches of a willow tree. The palm effect uses fewer, larger stars placed in straight lines. When ignited, these shoot out in bold, straight lines like palm fronds, while a thick trail of sparks follows the shell upward like a tree trunk. Some firework stars are designed to change shape mid-air. In a crossette effect, each star contains a small charge that explodes a second time, splitting the star into several smaller pieces that shoot off in different directions. This creates a crisscross effect as the fragments break apart and spread out.


How to Explore Firework Science with Kids
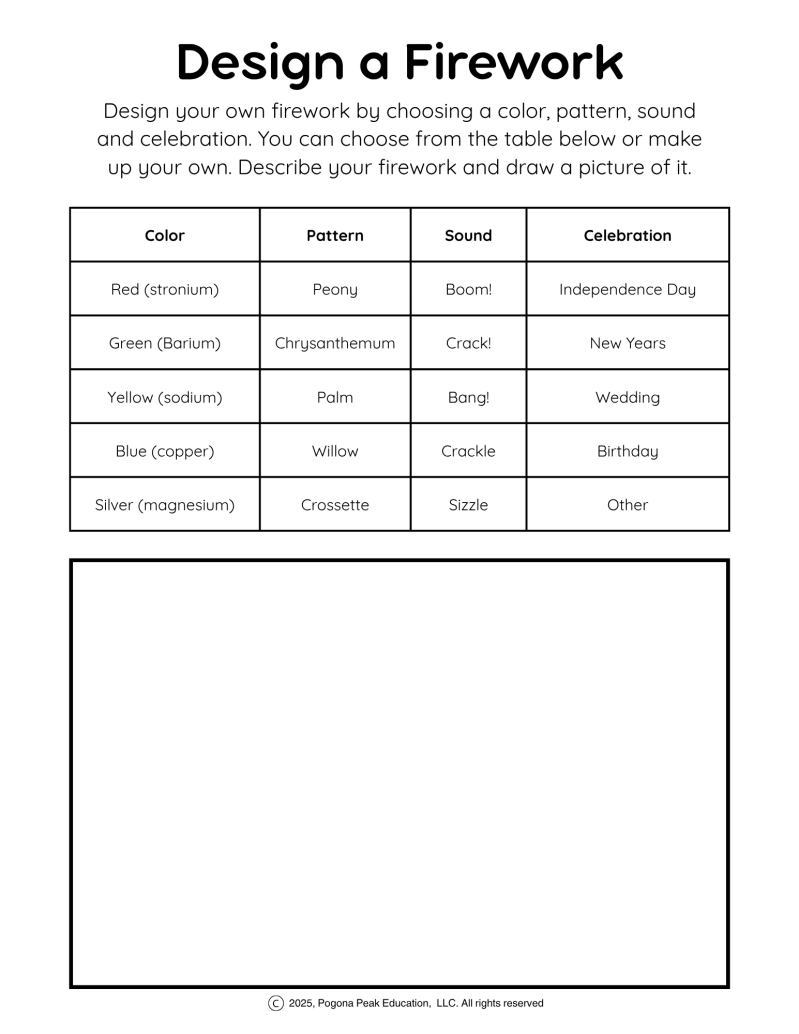
There are so many fun ways to explore fireworks with your kids beyond just watching them. You can challenge them to guess what elements might be making each color they see in the sky. You could talk about sparklers and how the metal burns when it reacts with oxygen, giving off light and heat. Or try a creative art project where your child draws and invents their own firework by choosing colors, shapes, and even the sound it might make. We have a FREE worksheet to design your own firework here.
We have a variety of fireworks-themed science experiments on our YouTube channel and many can be found in this previous blog post about 4th of July Science Activities.

If you're traveling on the road, or just looking for a no mess, print and go activity to learn about fireworks, check out our Science of Fireworks Activity Pack. It includes reading passages about the science of fireworks, coloring pages, mazes, word-searches, crossword puzzles, flash cards, and more!
What’s most exciting about fireworks is that they show science in action. Every flash of color is a chemical reaction. Every explosion is a demonstration of physics. Each carefully timed burst is an example of precise engineering. And behind every display is a mix of planning, creativity, and scientific know-how.



Add comment
Comments